WHO Warns on 3 Toxic Syrups in India

WHO Flags 3 Toxic Cough Syrups in India: Coldrif Linked to 22 Child Deaths in Madhya Pradesh
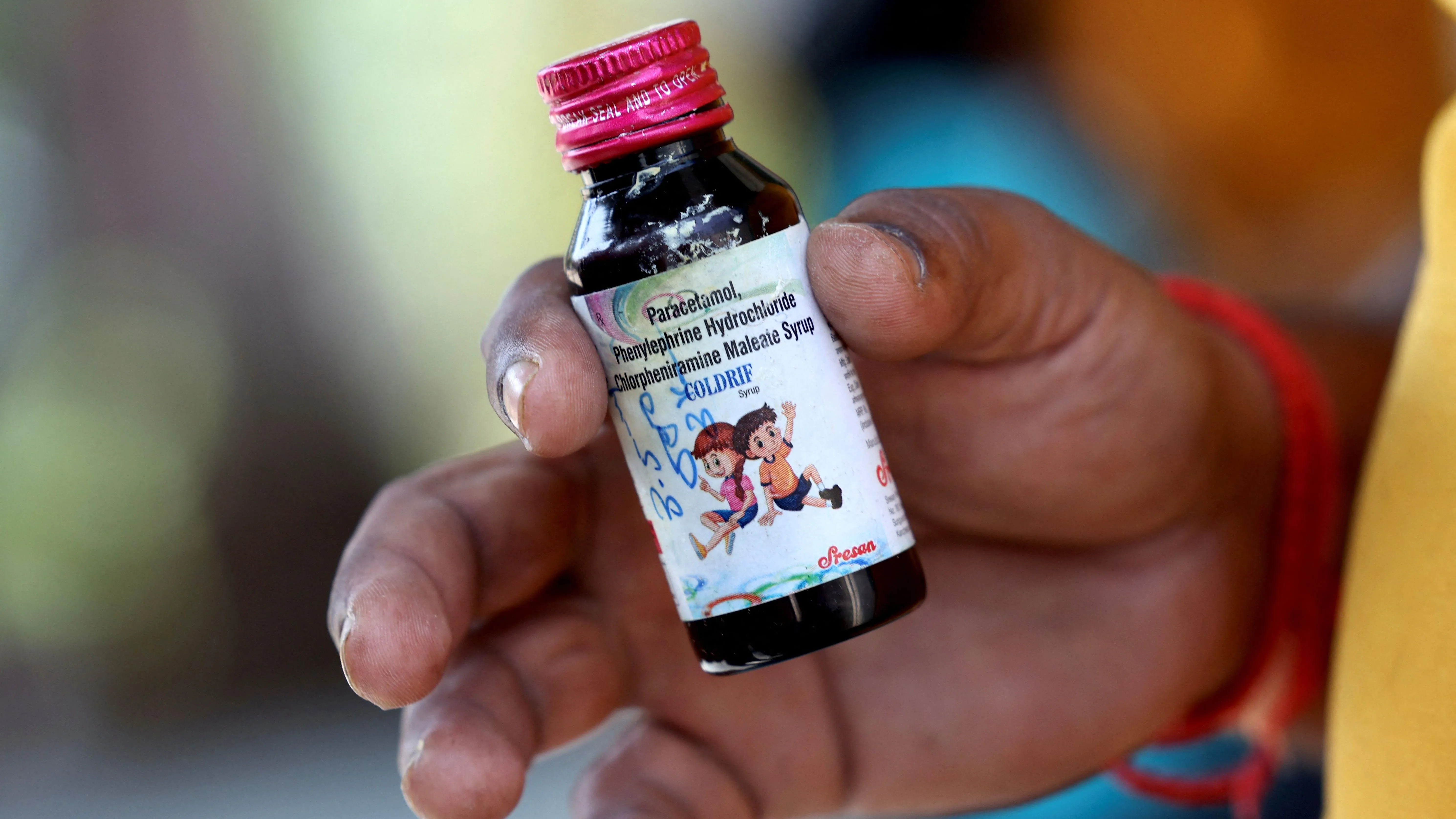
In a chilling alert that has sent shockwaves through India's healthcare landscape, the World Health Organization (WHO) has spotlighted three contaminated cough syrups circulating within the country, including the notorious Coldrif, amid a heartbreaking outbreak of child fatalities in Madhya Pradesh. The global body issued a medical product alert on October 10, 2025, identifying specific batches of Coldrif from Sresan Pharmaceuticals, Respifresh TR from Rednex Pharmaceuticals, and ReLife from Shape Pharma as laced with diethylene glycol (DEG)-a deadly sweetener surrogate responsible for the deaths of at least 22 toddlers in Chhindwara district. This Coldrif cough syrup warning WHO development underscores systemic lapses in pharmaceutical oversight, prompting urgent calls for nationwide recalls and stricter import-export scrutiny, as parents nationwide grapple with the terror of everyday remedies turning lethal.
The tragedy unfolded in Parasia village, where over 200 children under five fell ill after consuming Coldrif for common colds, with lab analyses revealing DEG levels at a staggering 48%-nearly 500 times the safe threshold of 0.1%. As grief-stricken families bury their young, the WHO's directive urges member states to investigate and report detections, emphasizing the syrups' potential for "acute kidney failure and irreversible organ damage." In India, where pediatric cough remedies dominate Rs 5,000 crore OTC sales annually, this scandal revives ghosts of 2022's global DEG crisis that felled 100+ Gambian children, exposing persistent vulnerabilities in low-cost formulations.
Sresan Pharmaceuticals, the Tamil Nadu-based culprit behind Coldrif, saw its manufacturing license yanked on October 5 by state drug controllers, with owner G. Ranganathan taken into custody on charges of culpable homicide. Parallel probes into Rednex and Shape Pharma have frozen their outputs, but experts warn of stockpiles in pharmacies, urging consumers to scan barcodes via the CDSCO's SUGAM portal for authenticity.
The Lethal Legacy of DEG: A Recurring Nightmare in Pharma
Diethylene glycol, a cheap antifreeze substitute for glycerin in cost-cutting formulations, has a sordid history of mass poisonings- from 1937's U.S. elixir deaths to Indonesia's 2022 toll of 200. In India, the 1986 Panacea scandal killed 15, while Uzbekistan's 2012 outbreak claimed 60. WHO's October 10 bulletin, disseminated via its global alert network, classifies these syrups as "substandard and falsified," with Coldrif's Batch L-24001 (expiry June 2026) topping the list for its egregious contamination.
Respifresh TR (Batch RF-145, Rednex Pharma, Gujarat) and ReLife (Batch RL-089, Shape Pharma, Himachal Pradesh) echo similar failings: Incomplete solvent testing and lax vendor audits, per CDSCO inspections. The Madhya Pradesh outbreak, erupting September 15, saw symptoms-vomiting, seizures, renal shutdown-mirror Uzbekistan's, with autopsies confirming DEG's nephrotoxic punch.
India's response, swift yet scrutinized, includes a nationwide ban on these batches and mandatory DEG assays for all liquid orals. Health Minister Mansukh Mandaviya's October 12 advisory to states prohibits under-two prescriptions, echoing 2023's FSSAI mandates, but pediatricians lament enforcement gaps in rural dispensaries stocking unverified generics.
Also Read: Army Kills 2 Terrorists in Kupwara
Government Crackdown: License Revocations and Arrests
Sresan Pharmaceuticals' downfall epitomizes regulatory retribution: The Sivakasi unit's license, issued 2018, was suspended September 25 post-initial tests, fully revoked October 5 under Drugs and Cosmetics Act Section 18(c). Owner Ranganathan, 52, faces FIRs in Chhindwara alongside supply chain accomplices, with raids yielding 5,000 contaminated bottles.
CDSCO's Assurance: No Exports, But Global Vigilance
Responding to WHO's September 28 query, CDSCO affirmed no exports of the tainted trio, corroborated by U.S. FDA scans showing zero shipments. Yet, the alert's ripple reaches 100+ nations, with Uzbekistan and Gambia recalling similar Indian generics-2022's legacy lingers, where 70% of probed syrups hailed from subcontinent labs.
- Batch Recalls: Coldrif L-24001, Respifresh RF-145, ReLife RL-089-destroy on sight.
- Testing Mandates: All pediatric liquids undergo DEG/GPMT assays quarterly.
- Vendor Audits: 500+ suppliers probed, with 20% blacklisted for adulteration.
Tamil Nadu's drug controller, post-Sresan, greenlit 50 surprise checks, unearthing solvent shortcuts in 15% of inspected units-a systemic scourge where profit trumps purity.
Broader Pharma Reforms on Horizon
Mandaviya's October 11 Rajya Sabha vow: "Zero tolerance for toxins," with Rs 200 crore for 50 new labs. FSSAI's 2026 roadmap eyes QR traceability for OTCs, while AYUSH integrates herbal alternatives like Tulsi drops, reducing synthetic reliance by 20% in trials.
Parasia's Heartbreak: 22 Tiny Lives Lost to a Bitter Pill
In Chhindwara's coal-dusted Parasia, the Coldrif saga shattered 22 families: Toddlers like 3-year-old Riya, who succumbed October 2 after days in ICU, her parents-daily wagers-hawking trinkets for dialysis. The village's ASHA worker, prescribing the Rs 50 bottle for "night coughs," now faces inquiry, emblematic of rural trust betrayed.
Survivors' scars: 150+ with acute renal failure, on hemodialysis at Nagpur AIIMS, costing Rs 5,000/session. Community vigils blend rage with resolve, with NGOs like Child Rights Initiative screening 1,000 kids for residuals. Madhya Pradesh's Health Department, deploying 50 teams, tallies 300 exposures, with 40% asymptomatic.
Globally, WHO's alert echoes Uzbekistan's 2022 woe, where Indian syrups tainted 100+-prompting export bans. India's 2023 response: 1,000+ raids, but 2025's lapse reignites calls for WHO's pre-shipment testing pacts.
Parenting in Peril: Safety Tips Amid Syrup Scares
For India's 25 crore under-fives, coughs are commonplace, but syrups spell danger: IAP guidelines nix under-twos meds, favoring saline nebulizers. Parents: Scan CDSCO's banned list via Sugam app; opt FSSAI-hallmarked brands like Benadryl; and consult pediatricians over ASHA generics.
Home remedies rise: Honey-lemon soothers (post-one-year) and steam inhalations cut visits 30%, per AIIMS studies. Schools in Chhindwara now mandate health cards, tracking OTCs-a model for 1 lakh anganwadis.
As WHO's siren wails, India's pharma behemoth-world's third-largest-must mend: From DEG detectors in every lab to export oaths, the path to purity passes through Parasia's pain.
In the crucible of crisis, vigilance vaccinates against villainy-lest another bottle betray.
Comment / Reply From
No comments yet. Be the first to comment!